Towards a mechanistic understanding of branching innovations in
plant evolution.
Jill Harrison and Yoan Coudert.
The conquest of land by plants was one of the most significant
events in our planet's history, and was underpinned by a series of innovations in
plant architecture. Amongst these, the innovation of branching stands out in
allowing plants to colonize new volumes of space in the subaerial environment.
Unlike most plants, living bryophyte representatives of the
earliest land plants have a biphasic life cycle with multicellular forms in
both the haploid (gametophyte) and diploid (sporophyte) life cycle stages. The
dominant photosynthetic phase of the life cycle is the gametophyte, and the sporophyte
typically comprises a single ephemeral stem capped in a spore-bearing
reproductive structure1.
Sporophytic branching forms are thought to have evolved once,
contributing to the radiation of our dominant vascular plant flora (c. 260,000
species). In contrast, distinct gametophytic branching forms have evolved in
each bryophyte lineage (c. 16,000 species)2.
Mosses are the most speciose bryophyte lineage (c. 10,000)2,3.
Although all mosses are relatively small, having leaves that are a single cell
thick, their branching habits are diverse and contribute to their ecology4 (Figure 1).
Figure 1: The diversity of branching forms in mosses. (A-E)
Photographs of herbarium specimens of (A) Braithwaitea
sulcata, (B) Hypopterygium arbuscula,
(C) Cyatophorum bulbosum, (D) Ancistroides genuflexa and (E) Hymenodontopsis stresemannii showing
variation in the vertical and radial distribution of lateral branches on the
leafy gametophyte. The distribution of the slender leafless sporophytic stems
also varies between species. In the species with erect gametophytic forms
(A-C), sporophytes are preferentially localised at the top of the shoot,
whereas in a species with a pendant form (D), the sporophytes are dispersed.
(E) has sporophytes with a lateral and basal position. Dr Yoan Coudert is
collaborating with colleagues at the Royal Botanic Garden, Edinburgh and the
Natural History Museum in London to characterise evolutionary trajectories
between these and other forms using a character mapping approach. Photos by Dr
Yoan Coudert, with thanks to NHM for access to specimens.
There is also an interplay between the gametophytic branching
habit and the arrangement of sporophytes on the stem, such that some forms have
a single sporophyte at the tip, some forms have a cluster of sporophytes
towards the top of the shoot, and others have sporophytes that are dispersed
over the plant.
The functional basis and significance of these differences in
architecture is not yet known.
Our recent work on the basis of branching patterns in the model
moss, Physcomitrella patens, provides
a starting point to identify the genetic mechanisms that underpinned the radiation
of branching forms in mosses5,6.
As there were no previous reports showing how branches arise in Physcomitrella, we started the project
by characterising initiation. Using SEM and histology, we found that branches
arise spontaneously from the epidermis with a patterned distribution6.
Data from flowering plants7, other mosses8, other labs9 and other unpublished projects in our lab led us to believe that
a hormonal interplay between auxin, cytokinin and strigolactone could
contribute to branching patterns.
We used a combination of computational modelling, genetics and
pharmacology to show that the integrated action of these three plant hormones
determines the distribution of branches up the gametophytic shoot6.
By varying the scope of contributions of each of hormone, we now aim
to reproduce the diversity of branching forms in mosses in silico, and will use modelling to generate predictions that
allow us to identify the basis of variation between species in future functional
work.
The distribution of branches around the shoot is a key component
of moss architecture that we have not yet taken into account (Figure 2), and several
studies have indicated that the epidermis of Physcomitrella may be the primary site of auxin response5,10,11.
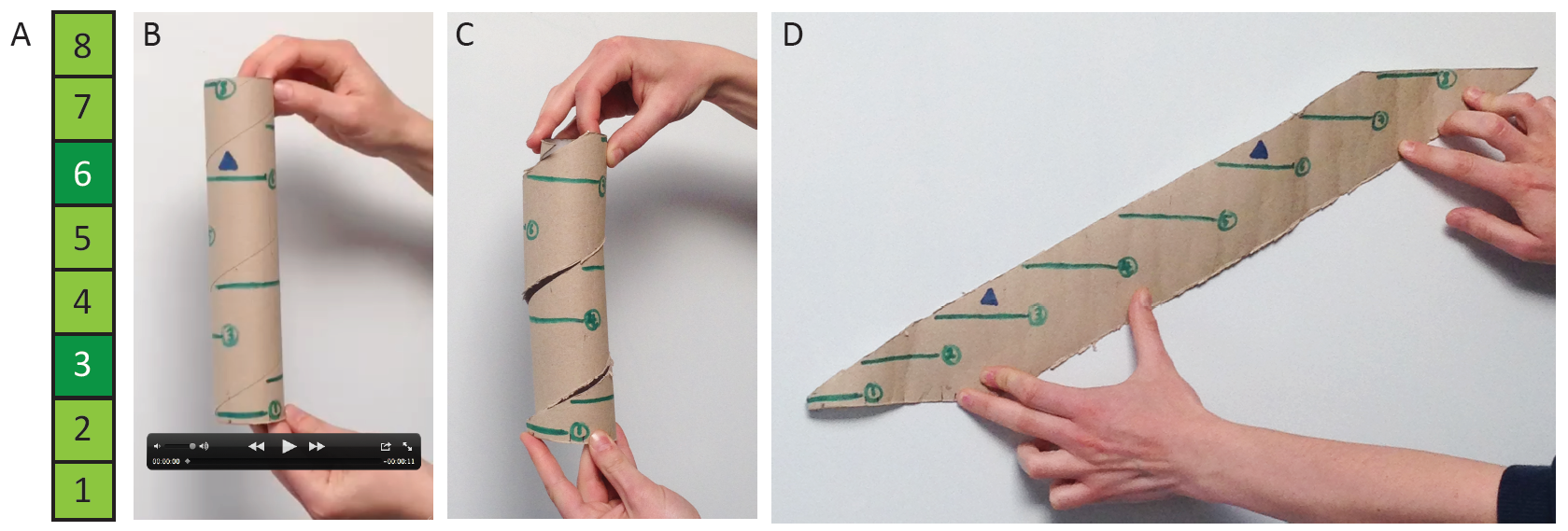
Figure 2: Gametophytic branching distributions (A) as represented
in Coudert et al. (2015), and (B-D) incorporating radial position. (A) Leaves
were removed in a numbered series from gametophytic shoots, and if a branch was
revealed, the position was recorded with dark green shading. (B) Movie of a
rotating kitchen roll holder with green lines representing leaves ascending the
shoot with a 137˚ divergence angle, and blue triangles representing a recorded
branch distribution. (C) Photograph showing a cut that allowed us to unravel
the kitchen roll holder to see (D) the radial distribution of branched
represented in 2D. Photos by Dr Jill Harrison and hands from Dr Yoan Coudert.
As branch
initiation is an epidermal phenomenon, we will adapt our 2D modelling approach
to analyse 3D branching architectures including radial patterning. We aim to analyse
the level and distribution of each plant hormone in relation to the branching
distribution with new fluorescent reporter systems in the future.
The work opens the door to mechanistic understanding of the
transitions in form that happened during the evolution of branching- one of the
defining features of our dominant land plant flora.
Further reading:
1 Langdale &
Harrison (2008). 'Developmental
changes during the evolution of plant form' in Evolving Pathways:
Key Themes in Evolutionary Developmental Biology (ed A. Minelli and G.
Fusco) p.299-315.
2 Shaw et
al. (2011). Bryophyte diversity and evolution: windows into the early evolution
of land plants. American Journal of
Botany 98, 353-369.
3 Laenen et al. (2014). Extant diversity of
bryophytes emerged from successive post-Mesozoic diversification bursts. Nature Communications 5, doi:doi:10.1038/ncomms6134.
4 La
Farge-England (1996). Growth form, branching pattern, and perichaetial position
in mosses: cladocarpy and pleurocarpy redefined. The Bryologist 99,
170-186.
5 Bennett et al. (2014). Plasma membrane-targeted
PIN proteins drive shoot development in a moss. Current Biology 24, 2776-2785.
6 Coudert et al. (2015). Three ancient hormonal
cues co-ordinate shoot branching in a moss. eLIFE,
doi:10.7554/eLife.06808.
7 Domagalska
& Leyser (2011). Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12, 211-221.
8 von
Maltzahn (1959) Interaction between kinetin and indoleacetic acid in the
control of bud reactivation in Splachnum
ampullaceum (L.) Hedw. Nature 183, 60-61.
9 Proust et
al. (2011). Strigolactones regulate protonema branching and act as a quorum
sensing-like signal in the moss Physcomitrella
patens. Development. 138, 1531-1539.
10 Bierfreund
et al. (2003). Use of an inducible reporter gene system for the analysis of
auxin distribution in the moss Physcomitrella patens. Plant Cell Reports 21,
1143-1152.
11 Jang et
al. (2011). RSL genes are sufficient for rhizoid system development in early
diverging land plants. Development 138, 2273-2281.
